Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -Ga
-Ga O
O (001)
(001)Tsai, Y. H.*; Kobata, Masaaki; Fukuda, Tatsuo; Tanida, Hajime; Kobayashi, Toru; Yamashita, Yoshiyuki*
Applied Physics Letters, 124(11), p.112105_1 - 112105_5, 2024/03
 Pt
Pt Al
Al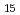
Ota, Kyugo*; Watabe, Yuki*; Haga, Yoshinori; Iesari, F.*; Okajima, Toshihiko*; Matsumoto, Yuji*
Symmetry (Internet), 15(8), p.1488_1 - 1488_13, 2023/07
Times Cited Count:1 Percentile:66.09(Multidisciplinary Sciences)Yuan, X.*; Hu, Q.*; Lin, X.*; Zhao, C.*; Wang, Q.*; Tachi, Yukio; Fukatsu, Yuta; Hamamoto, Shoichiro*; Siitari-Kauppi, M.*; Li, X.*
Journal of Hydrology, 618, p.129172_1 - 129172_15, 2023/03
Times Cited Count:0 Percentile:0(Engineering, Civil)Mohamad, A. B.; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko
Journal of Nuclear Science and Technology, 60(3), p.215 - 222, 2023/03
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Collaborative Laboratories for Advanced Decommissioning Science; The University of Tokyo*
JAEA-Review 2022-057, 98 Pages, 2023/02
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2021. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2020, this report summarizes the research results of the "Quantitative evaluation of long-term state changes of contaminated reinforced concrete considering the actual environments for rational disposal" conducted in FY2021. The present study aims to construct a database for quantitative prediction of contaminated reinforced concrete inside the reactor building. In FY2021, data on deformation and water movement caused by drying and reabsorption of mortar were obtained to evaluate the mesoscale cracking behavior of concrete. A rigidbody spring model was used to develop a program that can consider changes in concrete age and temperature, water, and stress conditions. To evaluate the long-term penetration behavior of radionuclides into the factual matrix, data on sorption …
 Cs NMR chemical shift of clay minerals via machine learning and DFT-GIPAW calculations
Cs NMR chemical shift of clay minerals via machine learning and DFT-GIPAW calculationsOkubo, Takahiro*; Takei, Akihiro*; Tachi, Yukio; Fukatsu, Yuta; Deguchi, Kenzo*; Oki, Shinobu*; Shimizu, Tadashi*
Journal of Physical Chemistry A, 127(4), p.973 - 986, 2023/02
Times Cited Count:1 Percentile:56.86(Chemistry, Physical)The identification of adsorption sites of Cs on clay minerals has been studied in the fields of environmental chemistry. The nuclear magnetic resonance (NMR) experiments allow direct observations of the local structures of adsorbed Cs. The NMR parameters of  Cs, derived from solid-state NMR experiments, are sensitive to the local neighboring structures of adsorbed Cs. However, determining the Cs positions from NMR data alone is difficult. This paper describes an approach for identifying the expected atomic positions of Cs adsorbed on clay minerals by combining machine learning (ML) with experimentally observed chemical shifts. A linear ridge regression model for ML is constructed from the smooth overlap of atomic positions descriptor and gauge-including projector augmented wave (GIPAW) ab initio data. The
Cs, derived from solid-state NMR experiments, are sensitive to the local neighboring structures of adsorbed Cs. However, determining the Cs positions from NMR data alone is difficult. This paper describes an approach for identifying the expected atomic positions of Cs adsorbed on clay minerals by combining machine learning (ML) with experimentally observed chemical shifts. A linear ridge regression model for ML is constructed from the smooth overlap of atomic positions descriptor and gauge-including projector augmented wave (GIPAW) ab initio data. The  Cs chemical shifts can be instantaneously calculated from the Cs positions on any clay layers using ML. The inverse analysis from the ML model can derive the atomic positions from experimentally observed chemical shifts.
Cs chemical shifts can be instantaneously calculated from the Cs positions on any clay layers using ML. The inverse analysis from the ML model can derive the atomic positions from experimentally observed chemical shifts.
Ohira, Saki; Iida, Yoshihisa
Proceedings of Waste Management Symposia 2023 (WM2023) (Internet), 10 Pages, 2023/02
The sorption distribution coefficient ( d) of niobium-94 (Nb-94) on minerals is one of the important parameters in safety assessment of radioactive waste disposal. In a previous study, the
d) of niobium-94 (Nb-94) on minerals is one of the important parameters in safety assessment of radioactive waste disposal. In a previous study, the  d values of Nb under alkali condition in the presence of Ca, were two orders of magnitude higher than those in the presence of Na. In this study, Nb sorption experiments were performed to reexamine the effect of Ca on Nb sorption on clay minerals, and blank tests were performed to check for precipitation formation. The results showed that the Nb sorption onto montmorillonite and illite, did not depend on the Ca concentration, and
d values of Nb under alkali condition in the presence of Ca, were two orders of magnitude higher than those in the presence of Na. In this study, Nb sorption experiments were performed to reexamine the effect of Ca on Nb sorption on clay minerals, and blank tests were performed to check for precipitation formation. The results showed that the Nb sorption onto montmorillonite and illite, did not depend on the Ca concentration, and  d values obtained in the presence of Ca were the same as those in the absence of Ca. A sorption model assuming sorption by complexation on the mineral surface was developed and then calculated using the geochemical calculation code. The model with the surface species X_ONb(OH)
d values obtained in the presence of Ca were the same as those in the absence of Ca. A sorption model assuming sorption by complexation on the mineral surface was developed and then calculated using the geochemical calculation code. The model with the surface species X_ONb(OH) and X_ONb(OH)
and X_ONb(OH)
 represented trends in the data obtained.
represented trends in the data obtained.
Miwa, Shuhei; Karasawa, Hidetoshi; Nakajima, Kunihisa; Kino, Chiaki*; Suzuki, Eriko; Imoto, Jumpei
JAEA-Data/Code 2021-022, 32 Pages, 2023/01
The improved model for cesium (Cs) chemisorption onto stainless steel (SS) in the fission product (FP) chemistry database named ECUME was incorporated into the severe accident (SA) analysis code SAMPSON for the more accurate estimation of Cs distribution within nuclear reactor vessels in the TEPCO's Fukushima Daiichi Nuclear Power Station (1F). The SAMPSON with the improved model was verified based on the analysis results reproducing the experimental results which were subjected to the modeling of Cs chemisorption behavior. Then, the experiment in the facility with the temperature gradient tube to simulate SA conditions such as temperature decrease and aerosol formation was analyzed to confirm availability of the improved model to the analysis of Cs chemisorption onto SS. The SAMPSON with the improved model successfully reproduced the experimental results, which indicates that the improved model and the analytical method such as setting a method of node-junction, models of aerosol formation and the calculation method of saturated CsOH vapor pressure can be applicable to the analysis of Cs chemisorption behavior. As the information on water-solubility of Cs deposits was also prerequisite to estimate the Cs distribution in the 1F because Cs can be transported through aqueous phase after the SA, the water-solubility of chemisorbed Cs compounds was investigated. The chemisorbed compounds on SS304 have been identified to CsFeO at 873 K to 973 K with higher water-solubility, CsFeSiO
at 873 K to 973 K with higher water-solubility, CsFeSiO at 973 K to 1273 K and Cs
at 973 K to 1273 K and Cs Si
Si O
O at 1073 K to 1273 K with lower water-solubility. From these results, the water-solubility of chemisorbed Cs compounds can be estimated according to the SA analysis conditions such as temperature in the reactor and the CsOH concentration affecting the amount of chemisorbed Cs.
at 1073 K to 1273 K with lower water-solubility. From these results, the water-solubility of chemisorbed Cs compounds can be estimated according to the SA analysis conditions such as temperature in the reactor and the CsOH concentration affecting the amount of chemisorbed Cs.
Li, N.*; Sun, Y.*; Nakajima, Kunihisa; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 11 Pages, 2023/00
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)During the Fukushima Daiichi nuclear power plant (1F) accident, an overwhelming amount of the cesium remaining in the pressure vessel could have been deposited onto 304 stainless steel (SS304) steam separators and dryers, both with large surface areas. During 1F's decommissioning, the deposited cesium is a safety hazard as it can generate radioactive dust. However, the cohesive and adhesive strengths of CsOH-chemisorbed oxide scales are yet to be defined. In this study, we investigated how CsOH-chemisorption affects the cohesive and adhesive strengths between oxide scales and SS304 substrates with a scratch tester. The scratch test results revealed that the cohesive strengths of the oxide scales decreased after CsOH-chemisorption, while adhesive failure could not be reached.
Saito, Tatsuo; Yamazawa, Hiromi*; Mochizuki, Akihito
Journal of Environmental Radioactivity, 255, p.107035_1 - 107035_14, 2022/12
Times Cited Count:0 Percentile:0(Environmental Sciences)The seasonal variation of dissolved U (DU) in Lake Biwa was reproduced by the following model and parameter research. The introduced models are the water-DU mass balance, and the ion exchange between UO
 and H
and H on the lakeshore soil. The optimized parameters were the CEC of the lakeshore, TU as the sum of DU and AU (soil adsorbed U), kads and kdes as the first order reaction rate coefficients during rapid soil adsorption and desorption of U, respectively. Tabulated by the chemical equilibria constituting DU and analyzed the contribution of each chemical species, it is shown that the seasonal variation of DU is caused by the seasonal variation of pH. A correction to the ion-exchange equilibrium to shift to first order rate reaction only when the daily AU ratio increased above kads or decreased below kdes, improved the reproducibility of DU measurements and reproduced the delay of the DU peak from the pH peak.
on the lakeshore soil. The optimized parameters were the CEC of the lakeshore, TU as the sum of DU and AU (soil adsorbed U), kads and kdes as the first order reaction rate coefficients during rapid soil adsorption and desorption of U, respectively. Tabulated by the chemical equilibria constituting DU and analyzed the contribution of each chemical species, it is shown that the seasonal variation of DU is caused by the seasonal variation of pH. A correction to the ion-exchange equilibrium to shift to first order rate reaction only when the daily AU ratio increased above kads or decreased below kdes, improved the reproducibility of DU measurements and reproduced the delay of the DU peak from the pH peak.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U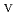
 Fe
Fe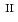
 U
U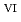 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Yakushev, A.*; Lens, L.*; D llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:9 Percentile:79.28(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Doi, Daisuke
Proceedings of 29th International Conference on Nuclear Engineering (ICONE 29) (Internet), 7 Pages, 2022/08
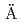 sp
sp Hard Rock Laboratory (Sweden)
Hard Rock Laboratory (Sweden)Soler, J. M.*; Meng, S.*; Moreno, L.*; Neretnieks, I.*; Liu, L.*; Kek l
l inen, P.*; Hokr, M.*;
inen, P.*; Hokr, M.*; 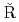
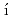 ha, J.*; Vete
ha, J.*; Vete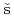 n
n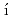 k, A.*; Reimitz, D.*; et al.
k, A.*; Reimitz, D.*; et al.
Geologica Acta, 20(7), 32 Pages, 2022/07
Times Cited Count:3 Percentile:57.97(Geology)Task 9B of the SKB Task Force on Modelling of Groundwater Flow and Transport of Solutes in fractured rock focused on the modelling of experimental results from the LTDE-SD in situ tracer test performed at the 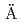 sp
sp Hard Rock Laboratory in Sweden. Ten different modelling teams provided results for this exercise, using different concepts and codes. Three main types of modelling approaches were used: (1) analytical solutions to the transport-retention equations, (2) continuum-porous-medium numerical models, and (3) microstructure-based models accounting for small-scale heterogeneity (i.e. mineral grains and microfracture distributions). The modelling by the different teams allowed the comparison of many different model concepts, especially in terms of potential zonations of rock properties (porosity, diffusion, sorption), such as the presence of a disturbed zone at the rock and fracture surface, the potential effects of micro- and cm-scale fractures.
Hard Rock Laboratory in Sweden. Ten different modelling teams provided results for this exercise, using different concepts and codes. Three main types of modelling approaches were used: (1) analytical solutions to the transport-retention equations, (2) continuum-porous-medium numerical models, and (3) microstructure-based models accounting for small-scale heterogeneity (i.e. mineral grains and microfracture distributions). The modelling by the different teams allowed the comparison of many different model concepts, especially in terms of potential zonations of rock properties (porosity, diffusion, sorption), such as the presence of a disturbed zone at the rock and fracture surface, the potential effects of micro- and cm-scale fractures.
Kakiuchi, Kazuo; Amaya, Masaki; Udagawa, Yutaka
Annals of Nuclear Energy, 171, p.109004_1 - 109004_9, 2022/06
Times Cited Count:4 Percentile:78.52(Nuclear Science & Technology)Soler, J. M.*; Neretnieks, I.*; Moreno, L.*; Liu, L.*; Meng, S.*; Svensson, U.*; Iraola, A.*; Ebrahimi, K.*; Trinchero, P.*; Molinero, J.*; et al.
Nuclear Technology, 208(6), p.1059 - 1073, 2022/06
Times Cited Count:4 Percentile:45.99(Nuclear Science & Technology)The SKB Task Force is an international forum on modelling of groundwater flow and solute transport in fractured rock. The WPDE experiments are matrix diffusion experiments in gneiss performed at the ONKALO underground facility in Finland. Synthetic groundwater containing several conservative and sorbing tracers was injected along a borehole interval. The objective of Task 9A was the predictive modelling of the tracer breakthrough curves from the WPDE experiments. Several teams, using different modelling approaches and codes, participated in this exercise. An important conclusion from this exercise is that the modelling results were very sensitive to the magnitude of dispersion in the borehole opening, which is related to the flow of water. Focusing on the tails of the breakthrough curves, which are more directly related to matrix diffusion and sorption, the results from the different teams were more comparable.
 and Eu
and Eu ions onto sedimentary rock in the presence of gamma-irradiated humic acid
ions onto sedimentary rock in the presence of gamma-irradiated humic acidZhao, Q.*; Saito, Takeshi*; Miyakawa, Kazuya; Sasamoto, Hiroshi; Kobayashi, Taishi*; Sasaki, Takayuki*
Journal of Hazardous Materials, 428, p.128211_1 - 128211_10, 2022/04
Times Cited Count:5 Percentile:62.11(Engineering, Environmental)The influence of humic acid and its radiological degradation on the sorption of Cs and Eu
and Eu by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs
by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs and Eu
and Eu ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu
ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu in the liquid phase was high, indicating that the complexing ability of HA to Eu
in the liquid phase was high, indicating that the complexing ability of HA to Eu was higher than that of HA to Cs
was higher than that of HA to Cs ions. Therefore, the sorption of free Eu
ions. Therefore, the sorption of free Eu would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
Sugiura, Yuki; Suyama, Tadahiro*; Tachi, Yukio
JAEA-Data/Code 2021-017, 58 Pages, 2022/03
Sorption behavior of radionuclides (RNs) in buffer materials (bentonites), rocks and cementitious materials is one of the key processes in a safe geological disposal of radioactive waste because RNs migration in these materials is expected to be retarded by the sorption process. Therefore, it is necessary to understand the sorption process and develop a database compiling reliable data and mechanistic/predictive models so that reliable parameters can be set under a variety of geochemical conditions relevant to a performance assessment (PA). For this purpose, Japan Atomic Energy Agency (JAEA) has developed the database of sorption parameters in bentonites, rocks and cementitious materials. This sorption database (SDB) was firstly developed as an important basis for the H12 PA of a high-level radioactive waste disposal, and have been provided through the Web. JAEA has continued to improve and update the SDB in the view of potential future needs of data focusing on assuring the desired quality level and testing the usefulness of the databases for possible applications to the PA-related parameter setting. This report focuses on updating of the sorption database (JAEA-SDB) as a basis of integrated approach for the PA-related distribution coefficient (Kd) setting and development of mechanistic sorption models. This report also includes an overview of the database structure and contents. Kd data and their quality assurance (QA) results were updated from literature collected with wider ranges. As a result, 8,503 Kd data from 70 references related to the above-mentioned systems were added and the total number of Kd values in JAEA-SDB reached 79,072. The QA/classified Kd data reached about 75.4% for all Kd data in JAEA-SDB. The updated JAEA-SDB is expected to make it possible to give a basis for the next-step PA-related Kd setting.
Collaborative Laboratories for Advanced Decommissioning Science; The University of Tokyo*
JAEA-Review 2021-047, 127 Pages, 2022/01
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2020, this report summarizes the research results of the "Quantitative evaluation of long-term state changes of contaminated reinforced concrete considering the actual environments for rational disposal" conducted in FY2020. The present study aims to construct a database for quantitative prediction of contaminated reinforced concrete inside the reactor building. In FY2020, in chapter 3.1, in order to obtain the data for the evaluation of mesoscale cracking behavior, the equipment for the making and the measurement of the test specimens were prepared, the evaluation method was confirmed, and preliminary experiments were carried out.